Sublimation is an intriguing yet somewhat mystifying concept to grasp. In short, sublimation refers to a solid turning directly into a gas without first melting into a liquid state.
But you may be wondering, why does this happen and is sublimation endothermic? That’s what we’ll explore in this article.
You May Also Like:
- Sublimation vs Vinyl: Choosing the Right Printing Method
- What Printers Can Be Used For Sublimation? (8 Picks!)
- What Is Sublimation Paper? (Types, Use Cases, etc)
- 6 Best Sublimation Inks In 2024 (I’ve Tested All !)
Is Sublimation Endothermic?
In thermodynamic terms, yes – sublimation is an endothermic process.
This means it requires an input of energy, specifically heat, to enable the phase transition from solid to gas.
The key takeaway is that energy must be absorbed by the sublimating substance for sublimation to occur.
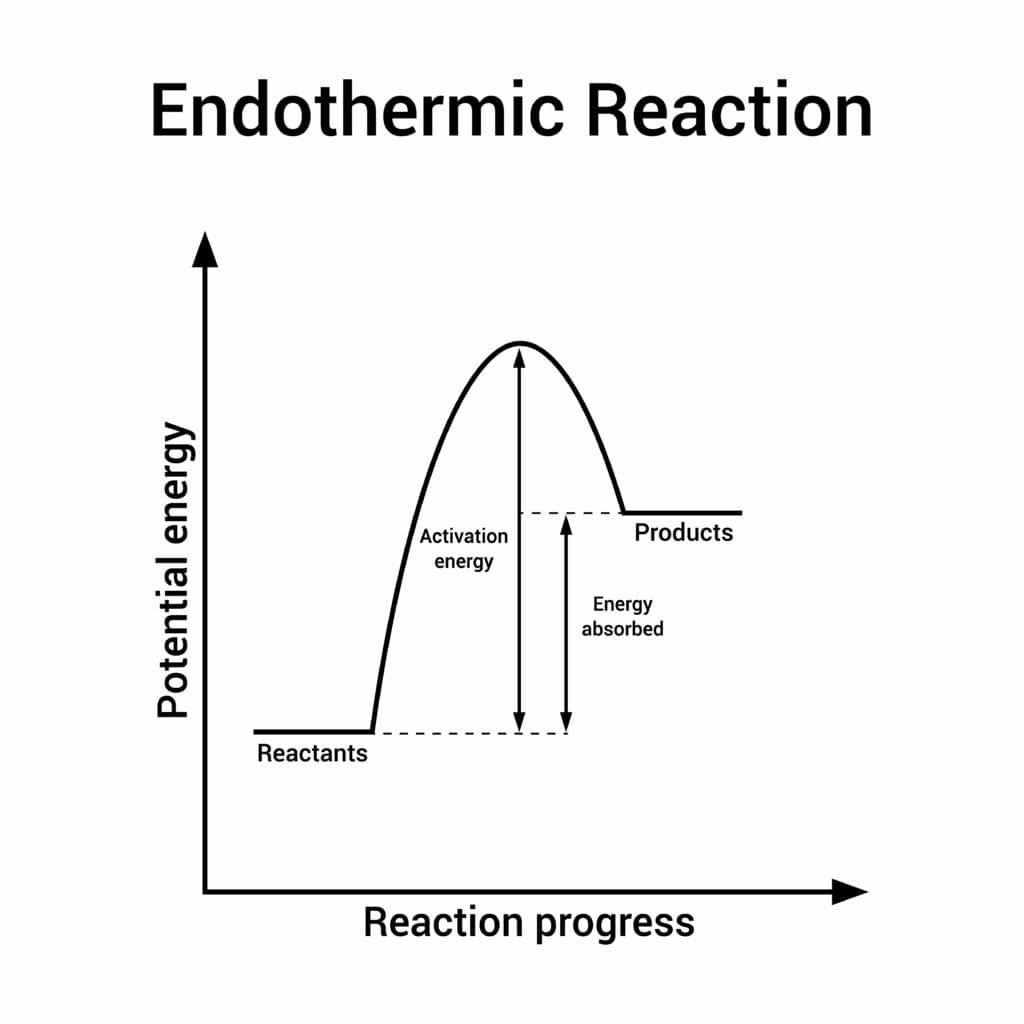
When a solid sublimates, the molecules need enough energy to completely break all the intermolecular attractions holding them in the rigid lattice structure.
Only then can they gain enough freedom of motion to behave like a gas. This energy is provided in the form of heat from the surroundings.
Think of sublimation as climbing up a hill, you need external energy to push you to a higher point. That uphill climb represents the breaking of intermolecular bonds.
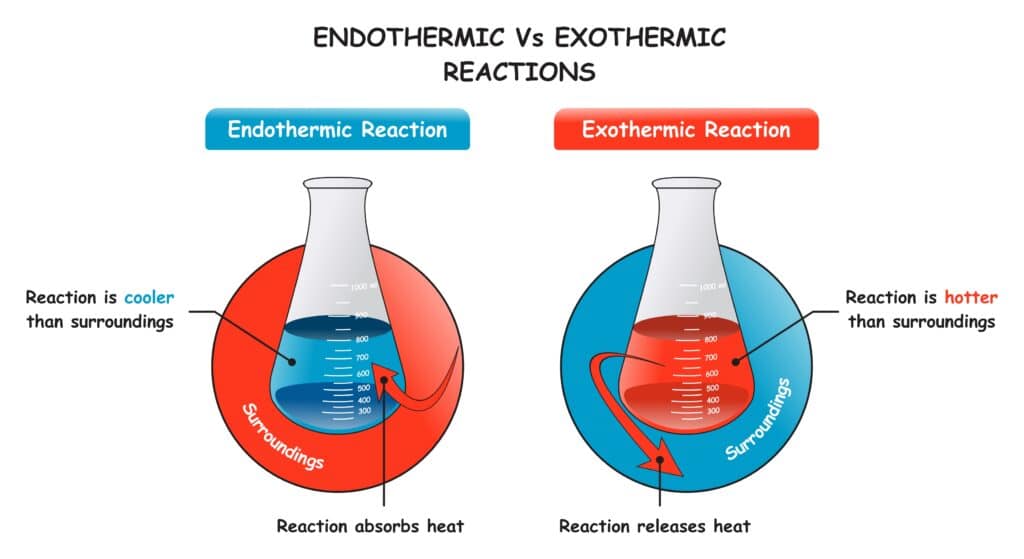
In contrast, exothermic processes release energy in the form of heat. So if sublimation was exothermic, it would get colder instead of hotter during the phase transition.
Why is Sublimation Not Exothermic?
While we have established sublimation requires heat intake and is endothermic, you may wonder – can it ever be exothermic instead?
Let’s analyze why heat release cannot occur:
1. Ordered Solids Have Lower Entropy than Gaseous State
Thermodynamically, solids possess lower entropy and molecular randomness compared to freely mobile gases. This is because solid particles exhibit an organized geometry and fixed lattice positions lacking freedom.
Gaseous molecules conversely translate and rotate arbitrarily across space with no restrictions.
This entropy increment from structured orderly crystals to wholly disordered gas particles makes the latter more stable energetically.
Consequently, the transition cannot be exothermic and give off heat spontaneously. Extra energy input becomes imperative to outweigh the stability of the solid state.
Therefore, sublimation cannot happen exothermically.
2. Breaking Binding Forces Outweighs Weaker Intermolecular Attractions Formed
Additionally, the various binding forces broken during sublimation like hydrogen bonds, ionic attractions, dipole forces, etc interactions possess far greater bond energies.
It is compared to new weak transient intermolecular interactions formed in the resulting gas.
For example, hydrogen bonds have bond strengths around 10-30 kJ/mol, whereas transient dipole-induced dipole attractions between gas particles have just 1-4 kJ/mol bond energy.
Enormous energy is required to break the stronger solid binding forces.
The weaker attractions subsequently formed cannot compensate for this breakdown through an exothermic heat release. The huge energy barrier to enable sublimation remains insurmountable without external heat.

3. High Kinetic Freedom of Gases Means No Exothermic Feasibility
Lastly, the enormous degrees of freedom, rotational, vibrational, and translational – gained by particles post-sublimation also support why the process cannot be exothermic.
With such high kinetic energy in the freely mobile gaseous state, no excess energy exists to be expelled exothermically.
Rather, the rotational chaos, increased vibrational amplitudes and high-velocity random collisions culminating from the heat absorption end up vitalizing the gaseous avatar, retaining any surplus energy within the system.
Eventually, this gets channeled into the pressure and temperature of the sublimed gas rather than manifesting as ejected heat.
Why Sublimation is an Endothermic Process
The endothermic nature of sublimation arises from various thermodynamic necessities:

1. Breaking Multiple Intermolecular Bonds
For sublimation to proceed, the many intermolecular attractive forces binding the particles in the solid lattice need to be overcome. This includes hydrogen bonding between molecules with hydrogen atom donors and acceptors.
Other bonds broken are van der Waals forces like dipole-dipole interactions between polar molecules and London dispersion forces between all atom types. Ionic compounds also have strong electrostatic attractions to be counteracted.
The energy required to break all these bonds is even greater than melting, where fewer bonds need dismantling to reach the liquid state.
So clearly, immense heat must be absorbed by the sublimating substance. Without sufficient thermal energy input across these various bonds, the phase change could not be enabled.
2. Facilitating a Major Increase in Molecular Freedom
Furthermore, as the solid transitions into the gas phase, a monumental increase in molecular degrees of freedom occurs.

Molecules twist, rotate, and translate completely independently instead of the rigid, organized structure in the previous solid lattice.
Achieving this hyperactive molecular mobility demands tremendous heat input. The added kinetic energy helps particles vibrate rapidly, collide forcefully, and escape their binding sites to behave as a gas.
3. Bypassing the Intermediate Liquid Stage
Additionally, the direct solid-to-gas transition in sublimation sidesteps the intermediate liquid stage that could have facilitated gradual, easier heating through incremental bond breaking.
With no stepwise phase change, the solid requires even higher magnitudes of heat absorption to reach the gaseous endpoint.
The liquid buffer would have allowed time for heat transfer while weakening intermolecular forces through increased particle motion.
This explains why more energy needs to be supplied for sublimation over other stepwise transitions like melting followed by vaporization.
In light of these thermodynamic requirements, extensive bond breaking, enormously raised molecular freedom, and the absence of an intermediate stage, the endothermic demand of sublimation becomes evident.
Supplying heat is mandatory to power this energy-intensive solid-to-gas conversion.
Endothermic Nature vs Exothermic Processes
The energy-absorbing essence of sublimation stands opposed to exothermic reactions that release heat instead. For example:

1. Freezing and Condensation are Exothermic
While sublimation requires heat input as an endothermic process, its reverse reactions (gas to solid) and condensation (gas to liquid) are exothermic. They give off considerable heat.
Why does this happen?
Because the molecules lose tremendous degrees of freedom condensing into the structured confines of a liquid or rigid lattice of a solid from the free-flowing gaseous state.
This drop in entropy and molecular motion liberates vast heat.
2. Contrast with Exothermic Photosynthesis
Additionally, compare the endothermic sublimation against exothermic photosynthesis in plants. This process utilizes sunlight energy to synthesize glucose from CO2 and water as raw materials.
The reaction gives off oxygen as a byproduct. Being an exothermic process, the breakage and formation of multiple chemical bonds here shed heat too.
This is the antithesis of the heat-absorbing nature of sublimation.
The thermodynamic essence varies enormously, sublimation endothermically removes heat for a physical change in state, while photosynthesis exothermically releases it for a chemical reaction.
Frequently Asked Questions
Below are a few frequently asked questions:
Why is sublimation not exothermic?
Sublimation cannot release heat or be exothermic because gases have higher enthalpy and entropy than solids.
The molecules require external energy to free themselves from the binding forces in an ordered solid.
Are both freezing and condensing exothermic?
Yes, both freezing (solid to gas) and condensing (gas to liquid) are exothermic phase changes.
As molecules lose degrees of freedom to become structured solids or liquids, heat gets liberated into the surroundings.
What are 2 examples of endothermic reactions?
2 examples of endothermic reactions requiring heat energy include:
1. Photosynthesis in plants
2. Decomposition of baking soda
Wrapping Up
We have thoroughly analyzed why sublimation acts as an endothermic process:
- It requires enormous heat to break multiple intermolecular bonds and allow the transition from an ordered solid to a freely mobile gaseous state.
- Additionally, bypassing the intermediate liquid stage means no gradual heating, necessitating even more heat.
- Unlike sublimation, reverse reactions like freezing and photosynthesis are exothermic, dissipating heat.
- Overall, sublimation demands the intake of thermal energies, cementing its endothermic nature.
With the thermodynamic principles governing sublimation’s hunger for heat now clear, think about endothermic applications from cryogenic cooling using solid CO2 to lyophilization technologies leveraging the solid-gas shift.
This can expand our capabilities for engineering phase change processes!
Let me know in the comment section if you need any clarification or have additional queries on this topic.